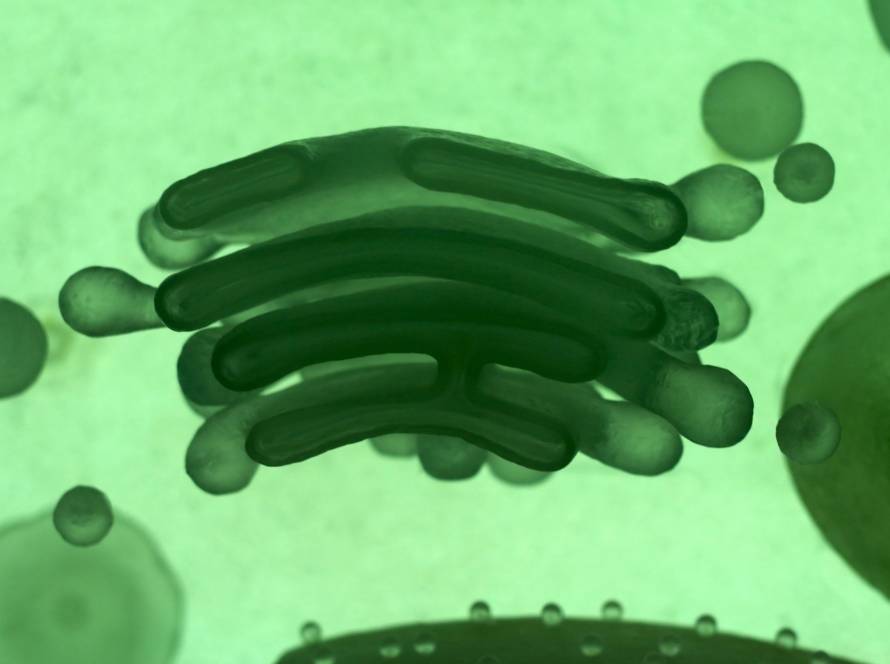
Mitochondria do much more than act as the “powerhouse of the cell.” These remarkable organelles perform many important functions that keep us healthy and help us live longer. They generate about 95% of cellular ATP (adenosine triphosphate) and work as the main energy producers in almost every cell in our bodies.
This detailed piece will get into what is the job of mitochondria, their origins and how they affect our health and aging. We’ll also look at proven ways to support mitochondrial function for better health and a longer life.
What role do mitochondria play in the body?
The primary job of mitochondria is to produce energy for the cell. They generate adenosine triphosphate (atp), the cell’s main energy currency, through a process called cellular respiration. Beyond energy production, mitochondria help regulate metabolism, control calcium levels and play a key role in programmed cell death (apoptosis).
They also influence aging and are involved in protecting cells from oxidative stress. Because of these vital roles, healthy mitochondria are essential for the function of high energy organs like the brain, heart and muscles.
What are mitochondria?
Rod-shaped mitochondria range from 0.5 to 10 μm in diameter. A distinctive double membrane system sets them apart from other cellular organelles. The outer membrane wraps around the organelles surface and contains special proteins called porins. The inner membrane creates numerous folds called cristae that extend into the interior matrix. This design increases the surface area for biochemical reactions substantially.
Only oxygen and ATP molecules can pass through the inner mitochondrial membrane, which creates a controlled environment for energy production. The space between these two membranes houses specific proteins that are essential for mitochondrial function.
The mitochondrial matrix contains a viscous fluid. This fluid is rich with enzymes, proteins, ribosomes, inorganic ions, mitochondrial DNA and organic molecules. These components are the foundations of the organelles metabolic activities.
Different cell types need different numbers of mitochondria. Human red blood cells have none, while liver cells and muscle cells might pack hundreds or even thousands. This matches each tissues energy needs throughout the body.
The main job of mitochondria: energy production
Mitochondrias main goal focuses on producing energy that powers almost all cell activities. These specialized organelles create adenosine triphosphate (ATP), the cell’s “energy currency”, through a sophisticated biochemical pathway that extracts maximum energy from nutrients.
How ATP is made through oxidative phosphorylation
The inner mitochondrial membrane hosts oxidative phosphorylation, which marks the final stage of cellular respiration. The process starts after glucose breaks down through glycolysis and the citric acid cycle. This breakdown produces high energy electron carriers.
The electron transport chain consists of protein complexes embedded in the inner mitochondrial membrane. Electrons move from high to low energy states through these complexes and release energy. This energy pushes protons (H⁺) from the matrix to the intermembrane space. The membrane now stores potential energy like a battery, thanks to this electrochemical gradient.
ATP production happens through chemiosmosis, a process that uses this stored gradient energy. ATP synthase (Complex V), a unique rotary motor protein, spans the inner membrane. Protons flowing back through this protein drive ADP and phosphate conversion into ATP. One ATP molecule needs about four protons to form.
Cells get much more energy this way compared to anaerobic methods. A cell without mitochondria would only get 2 ATP molecules from each glucose molecule through glycolysis. Mitochondrial oxidative phosphorylation produces about 30-32 ATP molecules per glucose molecule. This represents a 15-fold boost in energy extraction efficiency.
Why ATP is essential for cell survival
ATP works as the universal energy currency that drives nearly all cellular activities. Its phosphate bonds, especially between the second and third phosphate groups, store energy. Energy releases when ATP breaks down to ADP and phosphate. This powers many cellular reactions that wouldn’t happen otherwise.
Cells need a steady ATP supply to stay organized and functional. ATP powers these vital processes:
- DNA and RNA synthesis;
- Protein synthesis and folding;
- Active transport across membranes;
- Muscle contraction and cell movement;
- Intracellular signaling;
- Purinergic signaling between cells.
Modern animal cells would struggle with just anaerobic glycolysis if they didn’t have mitochondria. Complex multicellular organisms probably wouldn’t exist. The body can’t store much ATP, so mitochondria must keep making it for survival.
ATP’s high energy phosphate bonds create a very favorable free energy change during hydrolysis. This thermodynamic advantage lets ATP power many energy hungry processes throughout the cell. Our bodies use and remake their weight in ATP daily, which shows how crucial ongoing mitochondrial energy production really is.
Beyond energy: 10 essential functions of mitochondria
Beyond their main role in energy production, mitochondria have many vital functions that keep cells healthy and alive. These remarkable organelles work as versatile hubs that orchestrate key biological processes alongside ATP synthesis.
Regulation of calcium signaling
These cellular powerhouses act as calcium buffers to maintain cellular calcium homeostasis. The mitochondrial calcium uniporter (MCU) complex takes up calcium while the mitochondrial Na⁺/Ca²⁺/Li⁺ exchanger (NCLX) releases it. This calcium exchange happens at mitochondria associated membranes (MAMs), special contact points where mitochondria meet the endoplasmic reticulum. The calcium uptake by mitochondria directly boosts ATP production by activating three enzymes in the Krebs cycle matrix.
Control of cell death (apoptosis)
The cells programmed death process depends heavily on mitochondria. Death signals trigger them to release specific proteins from their intermembrane space that activate caspase proteases. Bcl-2 family proteins carefully control this process, known as mitochondrial outer membrane permeabilization (MOMP). Released cytochrome c combines with Apaf-1 to form a wheel-shaped apoptosome that triggers caspase-9 and later caspases-3 and -7, leading to cell breakdown.
Heat generation in brown fat
Mitochondria in brown adipose tissue create heat instead of ATP through non-shivering thermogenesis. This unique feature relies on uncoupling protein 1 (UCP1) that turns the proton gradient across the inner membrane into heat rather than ATP. Cold exposure activates brown fat to prevent hypothermia and uses up circulating glucose, fatty acids and other metabolites that may improve metabolic health.
Lipid and amino acid metabolism
These organelles break down fatty acids through beta-oxidation, which makes them vital for lipid breakdown. They work with lipid droplets and the endoplasmic reticulum to process lipids based on available nutrients. The mitochondrias role extends to all 20 amino acids that are either made or broken down within them. The matrix of mitochondria is where branched chain amino acids break down, which helps energy metabolism and creates new mitochondria.
Nucleotide synthesis and DNA repair
Mitochondria stand out from other organelles because they have their own DNA genome (mtDNA) and repair systems. Base excision repair (BER) is the main way they remove damaged DNA bases from oxidation, alkylation and deamination. DNA glycosylases work inside mitochondria to find specific damage. When repair isn’t possible, quality control processes like fusion, fission and mitophagy help remove damaged mtDNA beyond repair.
Signaling hubs of the cell
Mitochondria act as central signaling platforms that integrate cellular energy state, metabolite concentrations and upstream signals to maintain homeostasis. These regulatory hubs release proteins, lipids, metabolites and reactive oxygen species that work as signaling molecules. This complex communication network coordinates cell-fate decisions and metabolic adjustments based on changing cellular environments.
Reactive oxygen species (ROS) signaling
In stark comparison to this traditional view of ROS as harmful agents, mitochondrial ROS (mtROS) are now recognized signaling molecules vital for cellular adaptation. Mitochondria produce superoxide during oxidative phosphorylation that converts to hydrogen peroxide, the main signaling ROS molecule. This controlled ROS production affects several pathways:
- Activates hypoxia-inducible factors (HIFs) to regulate oxygen sensing;
- Promotes cell differentiation and wound healing;
- Regulates intracellular pH homeostasis;
- Improves immunity and pathogen clearance;
- Takes part in longevity-extending pathways.
MtROS signaling activates antioxidant defenses and creates balanced redox homeostasis that’s vital for cellular health.
Hormone production and steroidogenesis
Steroid hormone biosynthesis cannot happen without mitochondria. The cholesterol side chain cleavage enzyme (P450scc) lives in the inner mitochondrial membrane, making these organelles essential for starting steroidogenesis. Steroidogenic cells in adrenal glands, ovaries, testes and placenta have plenty of mitochondria for this specific purpose.
Mitochondrial dynamics (fusion and fission processes) directly control hormone production.
Immune system signaling
Mitochondria shape immune responses through multiple mechanisms. They release mitochondrial danger associated molecular patterns (DAMPs) that direct immune responses against pathogens. The mitochondrial antiviral signaling protein (MAVS) in the outer mitochondrial membrane coordinates antiviral responses.
MtROS substantially contribute to inflammasome activation. The NLRP3 inflammasome needs mtROS to produce inflammatory cytokines effectively. T cell activation also depends on mtROS from complex III, which shows mitochondria’s vital role in adaptive immunity.
Stem cell regulation and differentiation
Mitochondrial function shapes stem cell identity and fate decisions. Research shows that working mitochondria determine stem cells regenerative potential. Low ROS levels help maintain stem cell quiescence and self renewal, while higher ROS levels propel proliferation and differentiation.
Mitochondria undergo major changes in number, morphology and metabolism during reprogramming to induced pluripotent stem cells (iPSCs). They move from oxidative phosphorylation to glycolysis. This process reverses when cells differentiate and need more energy.
Support for cell division and growth
Mitochondria support cell division in several ways. They affect centrosome homeostasis and their dysfunction leads to centrosome overduplication and mitotic spindle defects. Mitochondrial fission and fusion dynamics affect cell division and disruptions cause chromosomal instability and G2/M arrest.
These organelles calcium buffering ability regulates cell division processes. Their skill at buffering calcium becomes especially important during mitosis when precise calcium signaling coordinates many division-related events.
What happens when mitochondria fail
Mitochondrial dysfunction demonstrates a progressive breakdown in the cells power generating capabilities. This breakdown triggers widespread effects throughout the body. The failures can stem from genetic mutations, environmental toxins or damage that builds up over time and ends up undermining cellular health and survival.
Mitochondrial dysfunction and aging
The mitochondrial theory of aging suggests that somatic mtDNA mutations create too many reactive oxygen species (ROS). These species damage mtDNA further in a continuous feedforward loop. The damage builds up over time and overwhelms cellular defenses. This contributes to the progressive decline and dysfunction of tissues. Research shows that mammalian species maximum lifespan associates with genetic control over oxygen utilization rates. These rates determine how much damage accumulates from free radicals in mitochondria.
Our mitochondrial dynamics become more unbalanced as we age. The decline in mitophagy with age stops the removal of damaged mitochondria. This leads to a buildup of mitochondrial damage and deterioration of cellular function So, Parkin deficiency with age makes abnormal mitochondria accumulate in mouse heart cells.
Links to neurodegenerative diseases
Neurodegenerative conditions show strong connections to mitochondrial failure. The central nervous system stays uniquely vulnerable to bioenergetic deficits. This vulnerability comes from its high metabolic activity, limited antioxidant defenses and mostly post-mitotic, irreplaceable neurons. Scientists first found complex I deficiency in the substantia nigra in Parkinson’s disease. Alzheimer’s disease shows early oxidative damage, reduced enzyme activities and cytochrome oxidase deficiency.
Huntington’s disease shows significant bioenergetic deficits through smaller and fewer mitochondria. ALS patients show mitochondrial damage including swelling, deformed cristae and respiratory chain defects. These often appear among the earliest signs of disease.
Cancer metabolism and oncometabolites
Cancer cells change their mitochondrial metabolism to support unlimited growth. Some tumors increase ROS generation, MAPK and EGFR signaling. This enables cancer causing pathways. Fumarate, succinate and 2-hydroxyglutarate (2-HG) build up abnormally. These true oncometabolites drive cancer transformation by blocking enzymes that control gene expression at the epigenetic level.
Cancer cells often resist mitochondrial mediated cell death. Many tumors show high mitochondrial membrane potential linked to increased glycolytic rates. Cancer stem cells can adapt their bioenergetic profiles based on tumor type and environment thanks to this metabolic flexibility.
Fatigue, metabolic disorders and chronic illness
Fatigue stands out as a key sign of mitochondrial dysfunction. People describe it as lack of energy, mental or physical tiredness, reduced endurance and slow recovery after activity. Rest often fails to relieve this fatigue, which is a defining symptom of mitochondrial disease.
Research has identified several mitochondrial markers linked to fatigue:
- Low carnitine levels associate with reduced functional capacity;
- Coenzyme Q10 deficiency consistently links to fatigue;
- Reduced ATP production and recycling contributes to chronic fatigue syndrome.
Multiple studies have found reduced cellular ATP levels. They also found disruptions in early TCA cycle stages in chronic fatigue patients. These bioenergetic failures translate to profound exhaustion in various chronic conditions.
Strategies to improve mitochondrial health and longevity
Good mitochondrial function is the life blood of healthy aging and disease prevention. We can improve our mitochondrial health through several proven strategies that work together to support these vital cellular components.
Exercise and mitochondrial turnover
Physical activity stands out as the most effective non-drug approach to mitochondrial health. When we exercise regularly, it promotes mitochondrial biogenesis by activating PGC-1α, which regulates this process. Both endurance training and high-intensity intervals trigger the p38-MAPK pathway and increase PGC-1α promoter activity. Exercise also activates mitophagy, which clears damaged mitochondria, through AMPK dependent mechanisms.
Nutritional interventions and fasting
Our diet substantially affects how mitochondria function. Calorie restriction, intermittent fasting and ketogenic diets improve mitochondrial respiratory function. A randomized clinical trial with obese participants showed these approaches changed monocyte metabolism from glycolysis to mitochondrial respiration while reducing inflammation. Our mitochondrial health improves especially during fasting as it triggers ketosis and switches metabolism from glycogen to fatty acids as main fuel sources.
Supplements with clinical evidence
These targeted supplements help mitochondrial processes:
- Coenzyme Q10: Essential for electron transport chain function;
- Alpha-lipoic acid: Mitochondrial cofactor with potent antioxidant properties;
- Carnitine: Helps fatty acid transport into mitochondria;
- N-acetylcysteine: CNS-penetrant antioxidant that improves glutathione synthesis.
Emerging therapies: mitophagy enhancers and NAD+ precursors
NAD+ precursors show promise for mitochondrial rejuvenation. Nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) increase NAD+ levels and activate SIRT1, which deacetylates PGC-1α to stimulate mitochondrial biogenesis. Higher NAD+ levels also promote mitophagy through multiple pathways and maintain mitochondrial network balance. Clinical research reveals NAD+ precursors improve muscle function, mitochondrial health and immune function. Mitophagy enhancers like Urolithin A also show notable benefits for muscle endurance in older adults.
Scientists and clinicians now recognize the deep connection between mitochondrial health and overall wellness. These organelles role in energy production, cellular signaling and metabolic regulation makes them the life blood of biological resilience.
Only when we are willing to see mitochondria as dynamic, responsive organelles can we develop effective health strategies. Supporting these vital structures through evidence based approaches influences many aspects of cellular function. This opens new paths to improved resilience, better metabolic efficiency and healthy longevity.